| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Choose from over 850 chemical products in chemical grades, sizes and concentrations to meet your needs. Carolina offers the highest quality kits for a hands-on approach within AP Chemistry classrooms. Ge oec 9800 user manual. Do not leak spoilers outside of the thread for chapter spoilers. Please use the button labeled spoiler or put the word 'spoilers' somewhere in the title for posts with spoilers in them.To mark spoilers in comments:Spoiler(/s 'spoiler-text')or!spoiler-text! Perrottetinene is a naturally occurring cannabinoid compound found in liverworts from the genus Radula native to Japan, New Zealand and Costa Rica, namely Radula perrottetii, Radula marginata and Radula laxiramea, along with a number of similar compounds. We offer the best exotic ethnobotanicals and psychoactive herbs such as Kratom, Kava Kava, Blue Lotus, and much more in resins, extracts, seeds, and tinctures.

| Clinical data | |
|---|---|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| ChemSpider | |
| Chemical and physical data | |
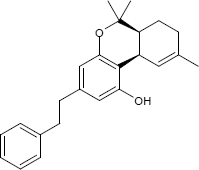
| Formula | C24H28O2 |
| Molar mass | 348.486 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Tutorial counter strike 1.6 for el capitan 10.11 bugatti. Perrottetinene is a naturally occurring cannabinoid compound found in liverworts from the genus Radula native to Japan, New Zealand and Costa Rica, namely Radula perrottetii, Radula marginata and Radula laxiramea,[1] along with a number of similar compounds.[2][3] Its chemical structure closely resembles that of THC, the main active component of marijuana. The absolute configuration of perrottetinene was established in 2008 by an enantioselective total synthesis.[4] In 2018, a study showed that perrottetinene is moderately psychoactive through activation of the cannabinoid receptor 1. The same study also reported reduced prostaglandin D2 and E2 brain concentrations in mice after perrottetinene administration.[5]
References[edit]

- ^Cullmann F, Becker H (1999-04-01). 'Prenylated Bibenzyls from the Liverwort Radula laxiramea'. Zeitschrift für Naturforschung C. 54 (3–4): 147–150. doi:10.1515/znc-1999-3-401.
- ^Toyota M, Kinugawa T, Asakawa Y (1994). 'Bibenzyl cannabinoid and bisbibenzyl derivative from the liverwort Radula perrottetii'. Phytochemistry. 37 (3): 859. doi:10.1016/S0031-9422(00)90371-6.
- ^Toyota M, Shimamura T, Ishii H, Renner M, Braggins J, Asakawa Y (October 2002). 'New bibenzyl cannabinoid from the New Zealand liverwort Radula marginata'. Chemical & Pharmaceutical Bulletin. 50 (10): 1390–2. doi:10.1248/cpb.50.1390. PMID12372871.
- ^Song Y, Hwang S, Gong P, Kim D, Kim S (January 2008). 'Stereoselective total synthesis of (-)-perrottetinene and assignment of its absolute configuration'. Organic Letters. 10 (2): 269–71. doi:10.1021/ol702692q. PMID18085788.
- ^Chicca A, Schafroth MA, Reynoso-Moreno I, Erni R, Petrucci V, Carreira EM, Gertsch J (October 2018). 'Uncovering the psychoactivity of a cannabinoid from liverworts associated with a legal high'. Science Advances. 4 (10): eaat2166. Bibcode:2018SciA..4.2166C. doi:10.1126/sciadv.aat2166. PMC6200358. PMID30397641.
Radula Perrottetii Buy